AFM and Confocal Fluorescence Microscopy can be Complementary Tools
Since its invention in 1986, atomic force microscopy (AFM) has been used to visualize the surface topographies of various samples by using the tip-sample interaction force. Due to its ability to operate in liquid environments, the AFM has had a significant impact in biological science. Figure 1 shows an AFM image of astrocytes, which are the major component cells of brain tissue. Astrocytes are fixed with 2% formaldehyde and 2% glutaraldehyde in PBS and imaged in phosphate buffered saline.

Figure 1. AFM image of astrocytes taken with XE-120 (75 μm scan size). Astrocytes are fixed with 2% formaldehyde and 2% glutaraldehyde in PBS and imaged in PBS.
AFM is a powerful tool for imaging the surfaces of cellular membranes, but biomedical research also requires observation of molecules inside the cells. In this aspect, confocal fluorescence microscopy can complement AFM imaging. Confocal fluorescence microscopy has become an essential tool in many areas of biological and medical research. Figure 2 shows confocal fluorescence microscopy image of astrocytes which is visualized with FITC-conjugated GFAP-specific antibody.
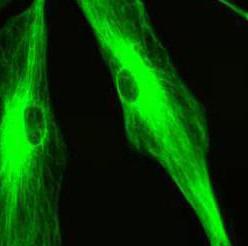
Figure 2. Confocal fluorescence microscopy image of astrocytes. GFAP is visualized with FITC-conjugated antibody (green).
Confocal fluorescence microscopy has various applications in biomedical research; for instance, FRET (fluorescence resonance energy transfer), FRAP (fluorescence recovery after photo-bleaching), FISH (fluorescence in situ hybridization), etc. Confocal fluorescence microscopy can be also extended to more advanced optical tools such as FLIM (fluorescence lifetime imaging microscopy), FCS (fluorescence correlation spectroscopy), TPE (Two-photon excitation) microscopy, SHG (second harmonic generation) microscopy, etc.
• AFM is a powerful tool for imaging the surface of cellular membranes.
• Confocal fluorescence microscopy obtains optical sectioning images inside the cell.
