Cell Study with Integrated Fluorescence Microscopy and SICM Study: H460
Brian Choi,Bio-application scientist
For more information, please contact app@parksystems.comSample courtesy: Hong-Bae Kim, Seoul National Univ.
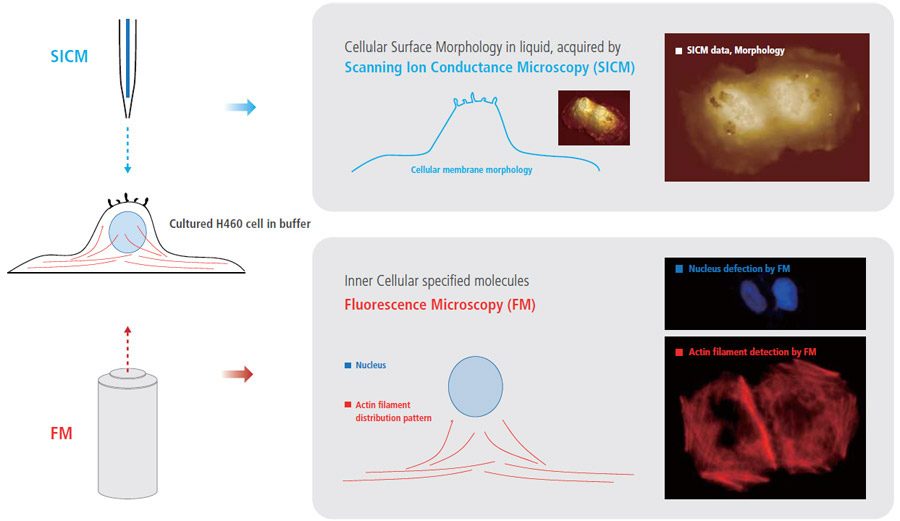
The combination of fluorescence microscopy and SICM techniques can create new benefits and can provide comprehensive information for cell biology studies that is not obtainable when using only one of those techniques. While monitoring the external cellular surface morphology with SICM, the internal cellular behavior can be observed by fluorescence microscopy. In this study, an electroporated H460 cell in buffer was observed using SICM and fluorescence microscopy. The irreversible electroporation was performed at 1 kV electric pulse, and the cell was fixed with 4 % paraformaldehyde. Then the cell was imaged with SICM imaging in 1x PBS. After staining the cell with rhodamine phalloidine (red) for actin filament identification in the cell, fluorescence microscopy images were captured.
- More Comprehensive Cell Biology Study by Integrating Fluorescence Microcopy with SICM
- While monitoring external cellular surface morphology with SICM, the internal cellular molecule can be observed by Fluorescence microscopy
Simultaneous Cell Observation of SICM (surface morphology) and Fluorescence Microscopy (actin filament structure) for Fundamental Cell Analysis
SICM imaged the treated H460 cell membrane topography first, and then the actin filament and nucleus images were acquired using fluorescence microscopy. The SICM image shows that the plasma membrane of H460 cell was physically damaged by electric pulse and many holes appeared on the surface. In the fluorescence microscopy image, the network structure of actin filaments inside the cell was clearly detected but were not damaged much.
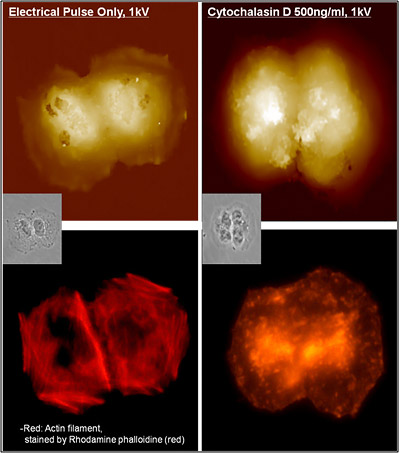
Cytochalasin (500 ng/㎖ ) treatment on H460 cell resulted to the same irreversible electroporation. Cytochalasin causes the actin filament to break off suddenly, by binding on it and blocking the polymerization. This action of cytochalasin on actin filament was distinctively detected by fluorescence microscopy observation. Interestingly, however, cytochalasin treated H460 cell does not show any holes on the cell membrane.
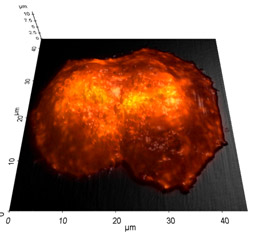 Image Overlay
Image Overlay
Fluorescence Microscopy Integration with SICM for Cell Study
Park Cell Analysis Systems
|
|
|
|
| Park NX12-Bio | Park NX10 | Park XE7 | |
| Scanning Ion Conductance Microscopy (SICM) | |||
| Atomic Force Microscopy (AFM) with liquid probe hand | |||
| Inverted Optical Microscopy (IOM) | |||
| Live Cell Chamber |
Irreversible Electroporation Mechanism Study: AGS cell
Brian Choi, Bio-application scientist
For more information, please contact app@parksystems.com
Sample courtesy: Hong-Bae Kim, Seoul National Univ.
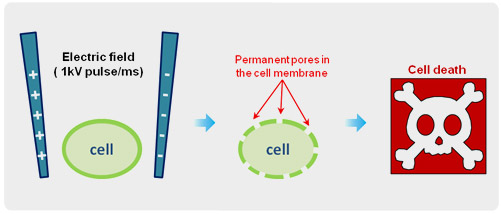
Irreversible electroporation (IRE) is a useful technique to remove cancer cells by applying ultra short electrical pulses directly. IRE creates permanent nanopores on the cell membrane that results to cell death, disrupting the cellular balance. Because of high tissue position selectivity and accessibility, IRE technique has been developing as a new promising cancer treatment tool in clinical studies. However, to date, little is known about its mechanism due to the lack of microscopic technique that can identify the nanopores in the cell membrane, generated by high voltage electrical pulses.
- Irreversible electroporation (IRE) is a promising cancer cell removal technique in tissue by applying electrical pulse.
- SICM is the only microscopy that can identify IRE-generated nanopores on the cell membrane in physiological conditions
 Irreversible Electroporation
Irreversible Electroporation
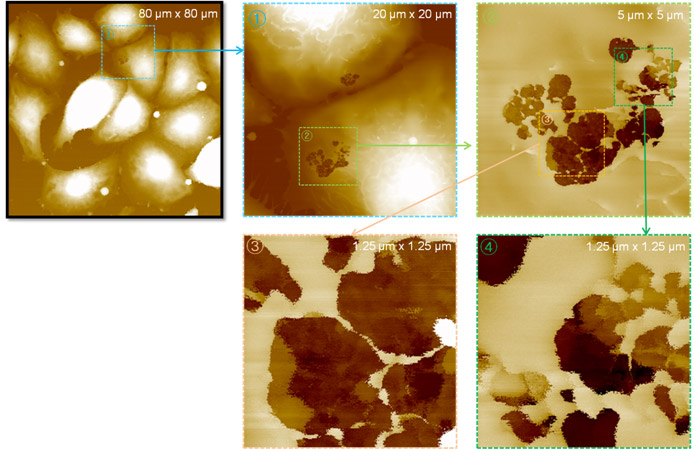
In this study, live gastric adenocarcinoma (AGS) cell was treated with 1 kV electrical pulse in 1 x PBS buffer for 1 ms. After 5 minutes of treatment, the cell was fixed in 4% paraformaldehyde and then the cell surface morphology was acquired using scanning ion-conductance microscopy (SICM) under physiological conditions. SICM detected many pores in the IRE treated AGS cell membrane. The details of the pore shapes are described in the range from micrometer to nanometer.
Irreversible Nanopore Detection on AGS cell by SICM
In zoomed-in image of 1.25 micrometer scan size passing the pores, some internal cell organelle features are shown in a few micrometer depth range.
Park Cell Analysis Systems

|

|

|
|
| Park NX12-Bio | Park NX10 | Park XE7 | |
| Scanning Ion Conductance Microscopy (SICM) | |||
| Atomic Force Microscopy (AFM) with liquid probe hand | |||
| Inverted Optical Microscopy (IOM) | |||
| Live Cell Chamber |
Direct Tissue Imaging in Liquid #2: Human Colon
Brian Choi, Bio-application scientist
For more information, please contact app@parksystems.com
Sample courtesy: Dr. Hyuk-soon Choi, Korea Univ. Hospitial
SICM can be used not only in cultured cell imaging but also in biological tissue studies. Tissue-level studies provide different insights compared to cultured cell studies. The naturally organized cells in tissue form a specialized structure for its dedicated function. The alteration of tissue structure can be an indication of losing its function and presence of disease. The phenotype of certain diseases at the tissue level with low resolution has been recorded for pathological purposes. Scanning electron microscopy (SEM) is frequently used as a tissue imaging tool due to wide - range observability and high- resolution imaging capability. However SEM cannot observe biological tissue in aqueous conditions, resulting in skepticism as to its efficacy in physiological studies.
- Observe the naturally organized cells in tissue structure for its dedicated physiological functions.
- Investigate the detail structure of human colon tissue by directly imaging tissue surface morphology in aqueous condition with SICM
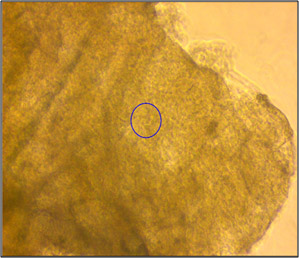 Inverted Optical View
Inverted Optical View
In this study, the morphology of human colon tissue in physiological conditions was visualized with SICM. Normal colon tissue taken from a human large intestine was used for the study. The tissue was soaked in pronase for two hours to remove mucus and fixed with 10% formalin before SICM investigation. The tissue was kept in 1 x PBS (phosphate buffer saline). Since the thickness of colon tissue is about a few hundred micrometers, as shown in the left image, it is not possible to observe surface morphology with an inverted optical microscope. However, SICM successfully imaged colon surface morphology without a complicated sample preparation process. The colon surface was imaged in various scan sizes, from 20 micrometers to five micrometers.
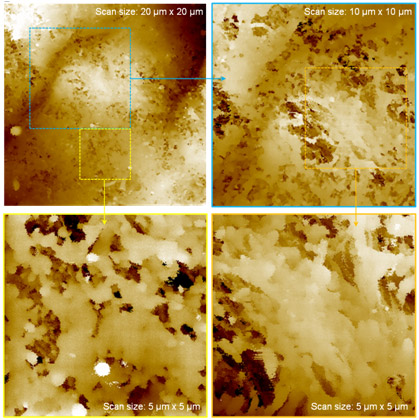 SICM, Human Colon Tissue
SICM, Human Colon Tissue
As revealed by the specific structure of human colon tissue in the 20-micrometer scan image, the morphology of organized single cells in human colon tissue was clearly observed in the five-micrometer scanned image. Mucus was removed from colon tissue surfaces with pronase treatment. SICM’s physiological tissue imaging offers a new possibility for understanding its functions in detail and relating those functions to structure. It also opens up a new way to investigate the real structural functioning patterns in various tissues. This result indicates that SICM imaging could be developed to provide diagnostic evidence for cancer tissue in the future.
Park Cell Analysis Systems

|

|

|
|
| Park NX12-Bio | Park NX10 | Park XE7 | |
| Scanning Ion Conductance Microscopy (SICM) | |||
| Atomic Force Microscopy (AFM) with liquid probe hand | |||
| Inverted Optical Microscopy (IOM) | |||
| Live Cell Chamber |
Direct Tissue Imaging in Liquid: Rat Tracheal cell
Brian Choi, Bio-application scientist
For more information, please contact app@parksystems.com
Data Reference: Prof. Ushiki and Dr. Nakajima, Niigata Univ.
The SICM image shown below is the world’s first observation of rat tracheal tissue in an aqueous environment. The luminal surface of the tracheal tissue was successfully imaged using Park SICM. For comparison, the same position of the tissue was imaged by scanning electron microscopy (SEM) after SICM measurement. To avoid structural damage to the sample, the tissue was very carefully dehydrated by a highly skilled researcher for SEM sample preparation. Highly ciliated cells are scattered on the luminal surface of the tracheal tissue. The hair-like cilia are easily waved by liquid flow in nature.
- Directly acquire physiological tissue images or single-cell images in an aqueous environment, without a laborious sample preparation procedure, with high resolution equal to SEM
- Cilliated cell in tracheal tissue imaging, not losing a single hair-like cilium in physiological conditions
Although the SICM pipette must approach this delicate surface repeatedly, every cilium in the imaged cell is visualized accurately, in contrast to the SEM image. Both ciliated and non-ciliated cells of tracheal tissue are pictured, especially the hair-like details of ciliated cells. This result is proof that scientifically explains with the SICM technique that in an aqueous environment we can directly acquire physiological cell and tissue images with a resolution equal to that of SEM.
 SICM, Ciliated Cell in Tracheal Tissue
SICM, Ciliated Cell in Tracheal Tissue
 Biological Tissue Preparation for SEM Imaging
Biological Tissue Preparation for SEM Imaging
The scanning electron microscope (SEM) has been an indispensable tool for cell biology due to its high-resolution cell imaging capability. To acquire an SEM image of a cell surface, a laborious sample preparation procedure, such as freeze-drying the whole cell and metal-coating the cell membrane, is inevitable. Because of this preparation, the cells are no longer considered living, but biologically dead. Moreover, the dead sample must stay in a vacuum chamber to ensure that electron paths remain straight; thus, SEM is not applicable under liquid conditions.
 Biological Tissue Preparation for SICM Imaging
Biological Tissue Preparation for SICM Imaging
In SICM imaging, the complicated sample preparation process of SEM imaging, which often causes physical damage of cellular and sub-cellular structures, is unnecessary. SICM frees researchers from the risk of artifacts and imaging failure. This technical advantage of SICM results in experimental high productivity for research and routine imaging needs.
Park Cell Analysis Systems

|

|

|
|
| Park NX12-Bio | Park NX10 | Park XE7 | |
| Scanning Ion Conductance Microscopy (SICM) | |||
| Atomic Force Microscopy (AFM) with liquid probe hand | |||
| Inverted Optical Microscopy (IOM) | |||
| Live Cell Chamber |
Monitoring Cytotoxicity: Epithelial cell
Brian Choi, Bio-application scientist
For more information, please contact app@parksystems.com
Data Reference: Gordon Jung, Application Scientist
Many toxins can cause acute epithelial inflammation and toxicity. However, the mechanism of such acute toxicity is difficult to elucidate and prove by experiment. Observing the negative effects of such toxicity at the single-cell level is a great help in explaining the mechanism in an intuitive manner. The accurate living cell membrane imaging of scanning ion conductance microscopy (SICM) can detect and visualize the negative effects in morphology occurring as a result of toxins.
- Accurate living cell membrane morphology imaging for visualizing single-cell cytotoxicity
- Cell membrane damage by exposure to Triton X is clearly monitored over time, which is not detectable in optical microscopic view
Cell Membrane Imaging After Detergent Exposure
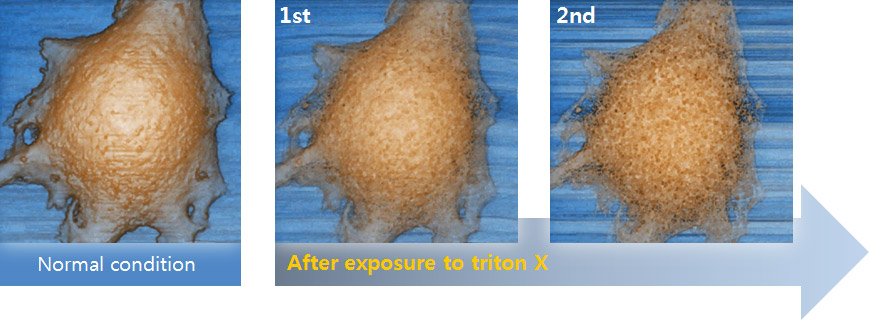
In this experiment, the early response of a living epithelial cell after exposure to Triton X (a nonionic detergent having highly toxic effects on cells) was investigated by SICM. Continuous SICM imaging revealed that cell membrane deformation occurred after Triton X exposure, and it showed the increased number and size of holes on the cell membrane.
Morphology Analysis in Line Data
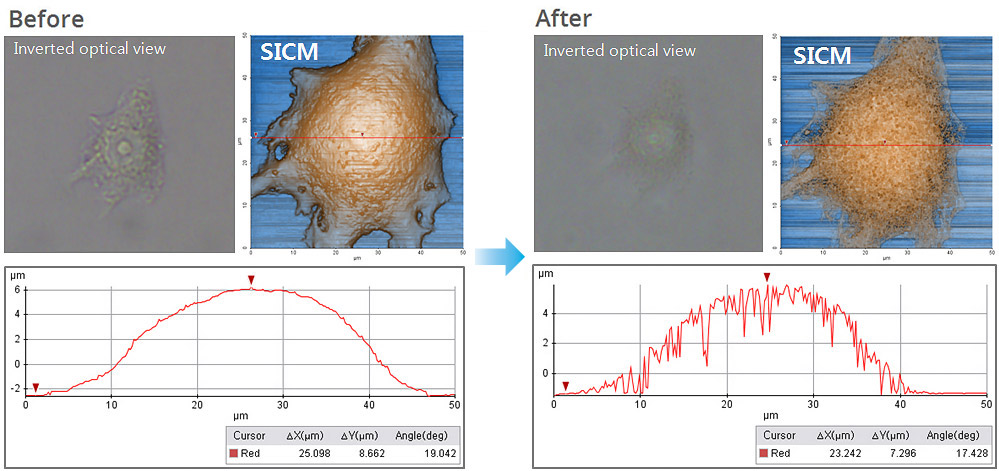
Cell membrane damage caused by Triton X is more distinctively identified by comparing the morphology analysis in single-line data (the damage is not distinguishable in inverted optical microscopic view). The line data analysis shows the depth and width of holes in the membrane in micrometers, while no hole structure appears in the line data of the cell image before Triton X exposure.
Park Cell Analysis Systems

|

|

|
|
| Park NX12-Bio | Park NX10 | Park XE7 | |
| Scanning Ion Conductance Microscopy (SICM) | |||
| Atomic Force Microscopy (AFM) with liquid probe hand | |||
| Inverted Optical Microscopy (IOM) | |||
| Live Cell Chamber |
