Introducing EC-AFM; Experimental setup and applications
Research Application Technology Center
Introduction
Electrochemistry is an essential study of industries such as batteries, fuel cells, semiconductors, etc. and, more broadly, can be adopted a wide range of research fields ranging from smelting, corrosion, polishing, coating of metals to the manufacture of various organic/inorganic compounds [1]. Electrochemistry examines the interactions of materials while tracking the movement of electrons when a potential is applied to electrodes in electrolyte [1], [2]. By combining cyclic voltammetry (CV) techniques with AFM surface imaging, electrochemical (EC)-atomic force microscopy (AFM) can be used to monitor the surface morphology and behavior of various electrode materials under electrochemical control [3].
EC-AFM is performed by connecting an EC cell and a potentiostat to enable monitoring of EC reactions based on AFM (Figure 1). A set of electrodes allows applying voltage to the sample where EC reactions can occur depending on the magnitude and direction of that voltage while an AFM setup measures the surface morphology and subsequent changes to it. The EC reactions can be studied both via the CV measurements as well as through AFM measurements that visualize any changes to the sample topography. In this application note, along with a brief introduction to Park EC-AFM, how EC-AFM is being used in the field of electrochemistry is explored with some examples.
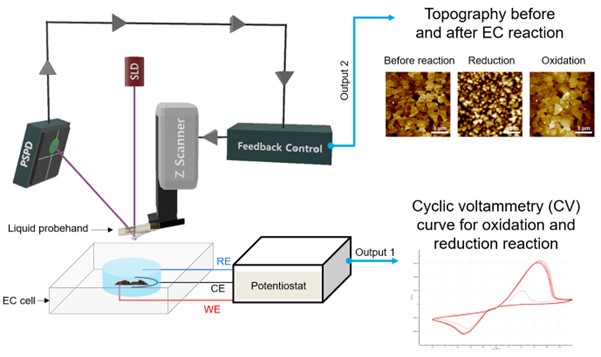
Figure 1. Schematic view of EC-AFM set up and examples of outputs (AFM images and CV curve)
Experimental setup
Liquid probehand
For AFM imaging in liquid, a specially designed liquid probehand (Figure 2(a)), should be used. This has two advantages: (1) to prevent the piezo actuator used to oscillate AFM probe from shorting out when submerged in fluid, and (2) a glass window eliminates unwanted reflections at the air-liquid interface so that only the reflected beam signal from the cantilever is detected.
An AFM probe is mounted on a Teflon-coated chip carrier to protect the EC cell from other unwanted electric signals and reactions that could affect the conditions of the electrolyte solution (Figure 2(b)).
EC cell
Park Systems’ EC cell is designed to simultaneously perform EC reactions while providing a suitable medium to image the topography of the sample surface. The EC cell is made from polyetheretherketone (PEEK), a high-performance thermoplastic material that is known for its excellent mechanical, thermal, and chemical properties. Three electrodes (working; WE, counter; CE, and reference electrode; RE) are integrated into the EC cell and connected to an external potentiostat to either apply potential bias while monitoring the current flow or apply a constant current while monitoring the bias to perform typical EC experiments (Figure 2(c)).
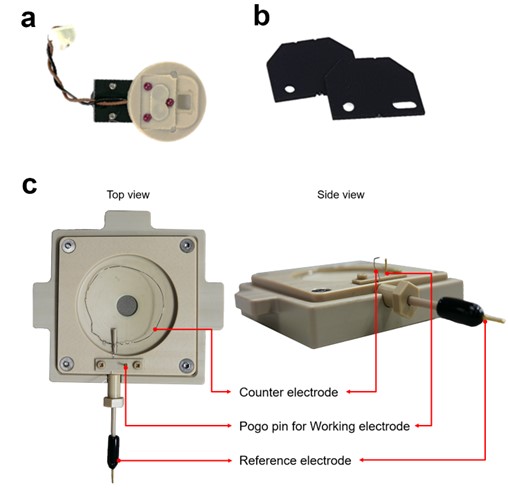
Figure 2. Experimental setup for AFM using EC cell. Park Systems liquid probehand (a), Teflon-coated chip carrier (b), EC cell with three electrodes (c).
Applications of EC-AFM
Copper deposition and dissolution on a Gold electrode surface
Figure 3 shows representative results of EC-AFM. The gold electrode surface morphology was monitored by AFM as copper (Cu) particles were deposited or dissolved according to the EC reaction on the gold (Au) surface. Cu deposition/dissolution on the Au surface by electrochemical response is fundamental research on electrochemistry and it has potential applications in several fields such as electroplating, corrosion protection, sensors and catalyst (electrochemical sensors, fuel cells, or electrocatalysis). Although simple and basic, the electrochemical behavior of metal deposition and dissolution processes is able to be leading to advancements in various electrochemical technologies. A thin gold (111) film evaporated onto a mica surface acts as the working electrode while reference and counter electrodes made from silver coated silver chloride (Ag/AgCl) and platinum iridium (Pt-Ir) wires, respectively. The electrolyte contained 0.1 mM CuSO4 in 50 ml of 0.01 mM H2SO4. Sulfuric acid was added to the solution for stabilization and to prevent the formation of copper precipitate. In all measurements, a voltage was applied to the Au (111) working electrode. By using AFM in Non-contact mode, on the Au surface before the EC reaction, bare Au film was observed, but when a voltage of -0.4 V was applied (Reduction), Cu particles were deposited on the Au surface. In the subsequent measurement, when a voltage of 0.1 V (Oxidation) was applied, it was confirmed that the Cu particles present on the Au surface were dissolved. These results are consistent with Eq. 1 and Eq. 2, it is a good example to check the result of deposition or dissolution of Cu particles according to the reaction of gaining (reduction) or losing (oxidation) Cu ion electrons with AFM [4].
Reduction reaction, Cu deposition: Cu2+(aq) + 2e- → Cu0(s)…… Eq. 1.
Oxidation reaction, Cu dissolution: Cu0(s) → Cu2+(aq) + 2e-……... Eq. 2.
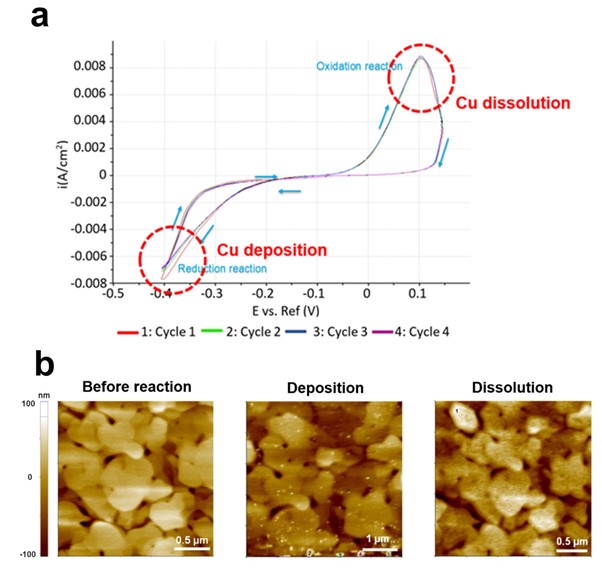
Figure 3. Cu particles deposition and dissolution on Au surface by EC reaction. CV curve indicates reduction and oxidation reaction at the applied -0.4 V and 0.1 V bias, respectively (a), AFM images at each step (b).
Monitoring of EC behavior of Li metal
Research on the behavior (nucleation, growth, stripping, and plating) of lithium (Li) metal under electrochemical control are being actively conducted as interest and utilization of Li ion/metal batteries have increased [5], [6]. Here, examples of studying the Li metal behavior according to the EC response of different conditions by EC-AFM are presented. Figure 4 depicts the deposition of Li particles on the bare Cu plate by the EC reaction by using EC-AFM. Cu foil was used as a working electrode, and Li metal was used as a reference and counter electrode. The electrolyte used in this measurement was 1M lithium bis-trifluoromethanesulfonimide (LiTFSI) in tetraethylene glycol dimethyl ether (TEGDME). Because Li is easily oxidized in ambient conditions, all measurements were performed in a dry room that maintains a humidity of less than 5 to 10%. Compared to the fresh bare Cu surface, some Li particles were deposited when a current of 0.1 mA/cm² (11.3 µA) was applied for 30 min. Here, the Cu plate was used as the cathode and a Li-containing electrolyte solution was used as the anode. When a current is passed through the system, Li ions are reduced at the cathode surface and deposit as Li metal.
Li1+(aq)+e-→Li(s)
This reaction involves the reduction of Li ions to Li metal, which is then deposited onto the Cu plate. The reaction is driven by the applied current, which provides the energy needed to reduce the Li ions and deposit the metal onto the cathode.
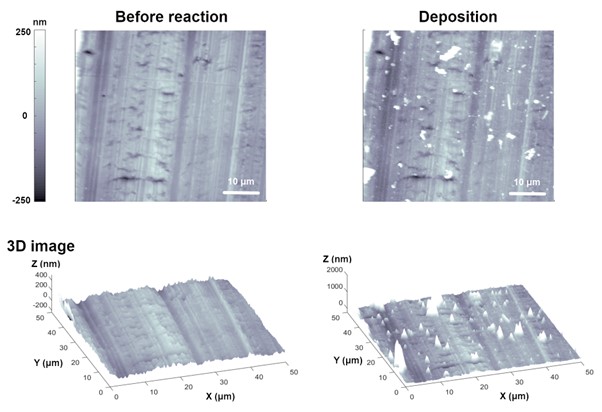
Figure 4. Electrodeposition of Li particles on Cu plate. Deposition of Li particles were observed when a current of 0.1 mA/cm2 (11.3 µA) was applied for 30 min.
Figure 5 is similar to Figure 4, but it is the result of measuring the behavior according to the EC reaction of Li on a Li metal plate rather than a Cu plate. The measurement conditions were the same, but Li metal was used as the working electrode. The surface morphology of the bare Li metal was characterized by AFM and then, after applying a constant current density of 2mA/cm² while monitoring the required bias at the same location for accurate comparison, it was confirmed that Li was stripped. After that, negative current density of -2mA/cm² was applied, and Li plating was confirmed. The potentiostat enabled tracking of the gradual increase in positive or negative bias to maintain a current density of ±2 mA/cm², confirming the stripping and plating reactions. Li stripping and plating refer to two processes in which Li ions are either removed from or deposited onto the surface of a Li metal, respectively. This process causes a decrease/increase in the concentration of Li ions in the Li metal, which results in a reduction/oxidation in the applied voltage. Both Li stripping and plating are important factors to consider in the design and operation of Li-ion batteries. If the stripping and plating processes are not controlled properly, they can lead to the formation of unwanted Li metal deposits on the electrode surface, which can cause battery failure or even safety issues such as thermal runaway.
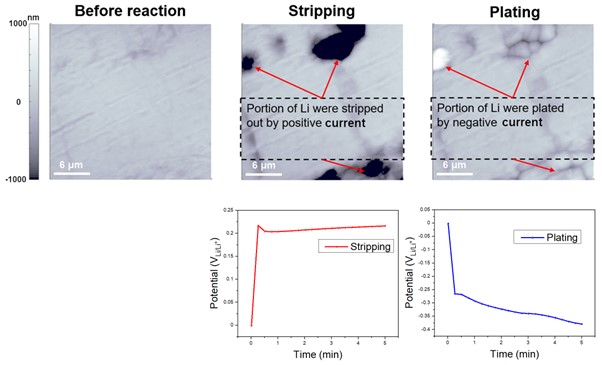
Figure 5. EC responses of Li stripping and plating. By allowing the current density to be maintained at ±2 mA/cm2, the potentiostat facilitated monitoring of the gradual changes in positive or negative bias, thus verifying the occurrence of the stripping and plating reactions.
Corroded metal surface by EC reaction
As a final application, the corrosion of the metal surface was measured by EC-AFM. The main purpose of this measurement is to confirm the corrosion of the metal surface according to the EC reaction in an aqueous solution containing salt, and secondly is to compare the degree of corrosion of metals treated with anticorrosive chemicals under the same conditions. In order to check the corrosion of the metal surface by EC response, after setting two metal types (bare cast iron and chemically treated cast iron) as working electrode, reference electrode and counter electrode were Ag/AgCl electrode and Pt-Ir wires, respectively, -0.3 V voltage was applied to investigate the change of the metal surface before and after the reaction. The electrolyte was used 3.5 w% NaCl.
As shown in Figure 6(a), compared to the bare cast iron surface (before the reaction), the corroded cast iron surface showed much rougher features. In general, when metal is in an aqueous solution, the anode and cathode are formed due to the non-uniformity of the metal surface, and corrosion proceeds by the local battery action. In addition, corrosion is accelerated as ions such as Cl- act locally on the surface and randomly destroy the surface film.
Anode reaction: M→M+ + e-
Cathode reaction: O2+ 2H2O + 4e- → 4OH-
As the above reaction proceeds, M+ ions increase on the metal surface, so M+Cl- is formed by combining with Cl- ions to maintain electrical neutrality. It is then hydrolyzed to HCl.
MCl+H2 O→MOH+HCl
As a result, the pH around the metal surface is lowered, and corrosion is accelerated.
Although there are various solutions to prevent corrosion of metal surfaces, corrosion inhibitors are treated on the surface to form a film such as a passive film on the surface to prevent corrosion. Figure 6(b) compares the degree of corrosion after EC reaction on the cast iron surface treated with corrosion inhibitor under the same conditions as Figure 6(a). Compared to the bare surface, it was confirmed that some debris were observed, but when compared to the cast iron surface without corrosion inhibitor treatment (Figure 6(a)), a clear difference is shown. The result of simultaneously measuring the EC response and the features of a metal surface corroded by an EC reaction is a good example of the usefulness of EC-AFM in metal corrosion research [7].
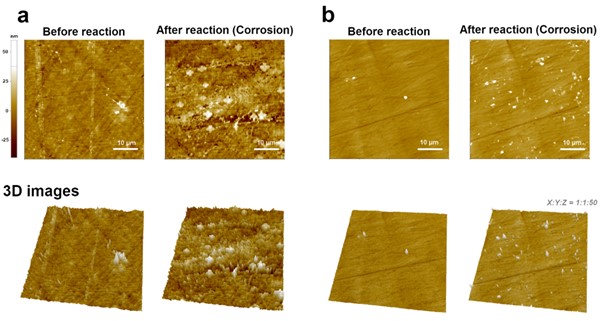
Figure 6. AFM images comparison between the fresh and corroded cast iron surface by EC reaction. Bare (a) and chemically treated (b) metal samples.
Conclusion
Electrochemistry is a crucial field of study with applications in various industries, including semiconductors, batteries, and fuel cells. It plays a vital role in understanding the interaction of materials and the movement of electrons during the application of potential to electrodes in electrolyte. EC-AFM is a noble technique in electrochemistry that combines electrochemical and AFM techniques to monitor EC reactions while measuring the sample's surface morphology. EC-AFM has become increasingly popular in the field of electrochemistry due to its ability to provide a more comprehensive understanding of the reactions occurring on the electrode surface.
Reference
[1]Glasstone, S. (2011). An introduction to electrochemistry. Read Books Ltd.
[2] Bagotsky, V. S. (Ed.). (2005). Fundamentals of electrochemistry. John Wiley & Sons.
[3] Manne, S., Massie, J., Elings, V. B., Hansma, P. K., & Gewirth, A. A. (1991). Electrochemistry on a gold surface observed with the atomic force microscope. Journal of Vacuum Science & Technology B: Microelectronics and Nanometer Structures Processing, Measurement, and Phenomena, 9(2), 950-954.
[4] Pineda, J. P., Leal, M., Pascual, G., Kim, B., & Lee, K. Electrochemical Atomic Force Microscopy: In Situ Monitoring of Copper Electrodeposition on Gold Surface.
[5] Yang, M., Liu, Y., Nolan, A. M., & Mo, Y. (2021). Interfacial atomistic mechanisms of lithium metal stripping and plating in solid‐state batteries. Advanced Materials, 33(11), 2008081.
[6] Gireaud, L., Grugeon, S., Laruelle, S., Yrieix, B., & Tarascon, J. M. (2006). Lithium metal stripping/plating mechanisms studies: A metallurgical approach. Electrochemistry communications, 8(10), 1639-1649.
[7] Chen, H., Qin, Z., He, M., Liu, Y., & Wu, Z. (2020). Application of electrochemical atomic force microscopy (EC-AFM) in the corrosion study of metallic materials. Materials, 13(3), 668.
